Pharmaceutical: Formulation & OSD


Oral Solid Dosage (OSD) products are created by combining the active pharmaceutical ingredient (API) with excipients to form a blend that can be compressed into tablets or filled into capsules. Unit operations include granulation (wet or dry), drying, milling, blending, compression, coating, encapsulation, and packing. In-process quality control (IPQC) ensures uniformity, potency, and adherence to quality specifications.
Key processes include:
- Wet granulation: Binderaddition, massing, drying in fluid bed dryer (FBD), and final blending.
- Dry granulation: Rollercompaction and milling without solvents or water.
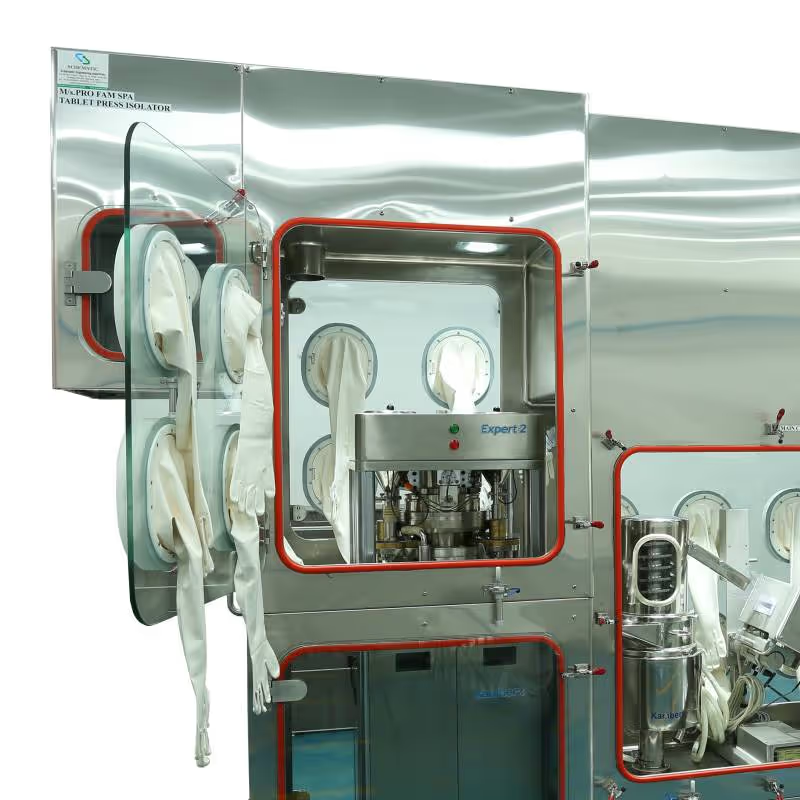
- Compression & coating: Contained tablet presses and coaters for safe, efficient operation.
- Encapsulation & packing: Capsule filling, blister packing, and bottle filling under containment.

High-potency APIs (HPAPIs) require robust containment to ensure operator safety and prevent cross-contamination. Schematic’s isolators maintain controlled airflow, pressure differentials, and safe-change filtration systems, ensuring clean, ergonomic workspaces while simplifying cleaning and validation.
Key containment features:
- OEB 4–5 containment capability
- Negative or positive pressure operation as needed
- HEPA safe-change filtration and CIP/WIP integration
- ISO 5–8 cleanroom compatibility
Common systems include:
- Granulation trains — high-shear granulators, mills, and blenders.
- Fluid bed dryers — efficient moisture removal with contained air handling.
- Tablet presses and coaters — precision compression and uniform coating under isolation.
- Capsule filling and blister lines — contained packaging solutions.
- Sampling, weighing, and sifting units — ergonomic dispensing with repeatable containment.

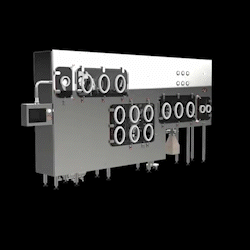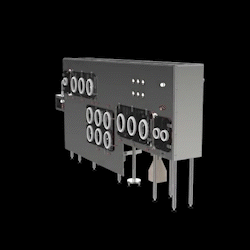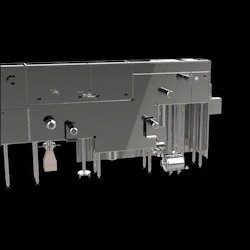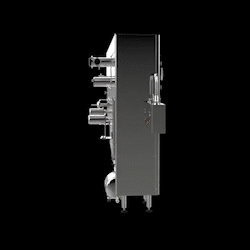
Schematic Engineering offers a full range of containment solutions that integrate seamlessly with OSD equipment. These include modular and custom isolators designed for containment, process visibility, and regulatory compliance.
Product families include:
- Sampling, Dispensing & Sifting Isolators — for accurate, safe batch initiation.
- Granulation Line Isolators —integrating RMG, mills, blenders, and FBDs under containment.
- Fluid Bed Dryer Isolators — for safe drying of granules and intermediates.
- Tablet Press Isolators —adaptable to all leading tablet press models.
- IPQC Isolators — ergonomic environments for in-process quality control.
- Tablet Coating Glove Boxes — for contained coating and sampling.
- Capsule Filling and Blister Packing Isolators — for secure downstream processing.
- Bottle Filling Line Isolators —for contained solid and liquid primary packaging.
- Pack-Off Isolators — for safe product discharge and sampling with liner systems.

The engineering highlights of Schematic's Formulation and OSD solutions include;
- 316L stainless steel internal surfaces, 304 external panels
- PLC / HMI controls with alarms and data logging
- Auto pressure testing to ISO 10648-2
- Safe-change HEPA filtration systems
- CIP / WIP ready with wash utilities and drain management
- Ergonomic glove ports and customizable integration with user equipment
- Customised configuration to suit the clients requirements
- Ergonomic